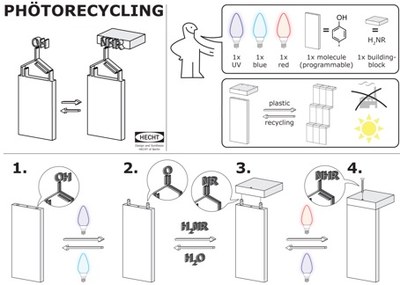
Shining new light on sustainable chemistry
A programmable molecule in combination with a specific light
sequence allows for bond formation (UV and red light; 1. to 4.)
or scission (UV and blue light; 4. to 1.) with molecular
building-blocks. Visualization: Michael Kathan.
From a chemist’s perspective, molecules are tiny building-blocks that can be interconnected via chemical reactions to give a large variety of materials for plethora of applications. According to this modular principle, the synthesis of very robust plastics, for instance, requires molecules, which are able to form strong bonds. This, however, also means that the recycling of such materials is quite problematic, since the cleavage of strong bonds requires a lot of energy, rendering the recovery of the initial building blocks rather difficult or even impossible.
A research team from the Humboldt-Universität zu Berlin has tackled this problem by designing a special molecule, which can be programmed with light of different colors to drive or reverse specific chemical reactions. This enables making and breaking of connections on the molecular scale, independent of the strength of the chemical bonds or the inherent preference of the underlying reaction.
“The working principle of our system is quite similar to the one of ready-to-assemble furniture” explain Michael Kathan and Fabian Eisenreich, the two first authors of this study. “We are able to repetitively assemble or disassemble molecular architectures, by using red and blue LEDs as tools instead of a hammer and screw-driver.” The discovery has great potential for the development of sustainable materials, as light-driven recovery of individual molecular building-blocks allows for recycling of yet non-recyclable plastics without compromising on color, quality, or shape.
Publication
“Light-driven molecular trap enables bidirectional manipulation of dynamic covalent systems”
Authors: Michael Kathan, Fabian Eisenreich, Christoph Jurissek, Andre Dallmann, Johannes Gurke, and Stefan Hecht
Published in: Nature Chemistry (2018), DOI: 10.1038/s41557-018-0106-8
Further Information
Contact
Prof. Stefan Hecht, Ph.D.
Humboldt-Universität zu Berlin
Department of Chemistry & IRIS Adlershof
Phone: +49 (0)30 2093-7365
sh@chemie.hu-berlin.de